


However, later it was concluded that the paddle wheel turned not due to the momentum of the particles (or electrons) hitting the paddle wheel but due to the radiometric effect (it is the repulsive force between two surfaces maintained at different temperatures).
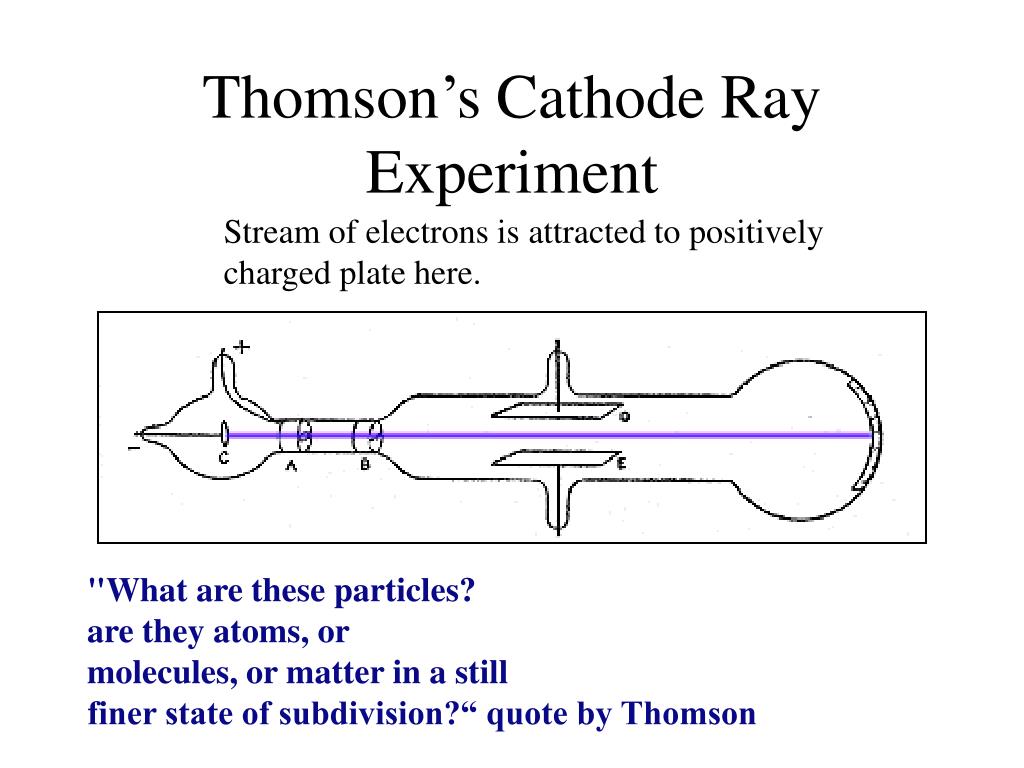
Crookes concluded at the time that this showed that cathode rays had momentum, so the rays were likely matter particles. The paddlewheel turned in a direction away from the cathode side of the tube, suggesting that the rays were coming from the cathode. So you should place it different tube to get the paddle wheel move by anode or cathode rays.Ĭrookes put a tiny vaned turbine or paddlewheel in the path of the cathode rays, and found that it rotated when the rays hit it. However, the tube must be set up quite differently from the usual cathode ray set-up in order to detect canal (or anode) rays. When high voltage is applied on electrodes the cathode emits electrons which collide with the residual gas knocking of electron from that gas molecule which travel towards the anode and the positively charged gaseous atom gaseous atom moves back to cathode which is anode rays. It is produced it the middle of the tube. In addition, the experiment could describe characteristic properties, in essence, its affinity to positive charge, and its charge to mass ratio. Thomson, is one of the most well-known physical experiments that led to electron discovery. Actually anode rays are not emitted from anode and reaching cathode. The experiment Cathode Ray Tube (CRT) conducted by J.


 0 kommentar(er)
0 kommentar(er)
